N and P have 5 valence level electrons so they will likely gain 3 electrons resulting in a 3- charge. The Periodic Table Electron Configuration Chemical Bonding Lecture 7.

Writing Electron Configurations Using Only The Periodic Table Youtube
This activity supports students understanding of.
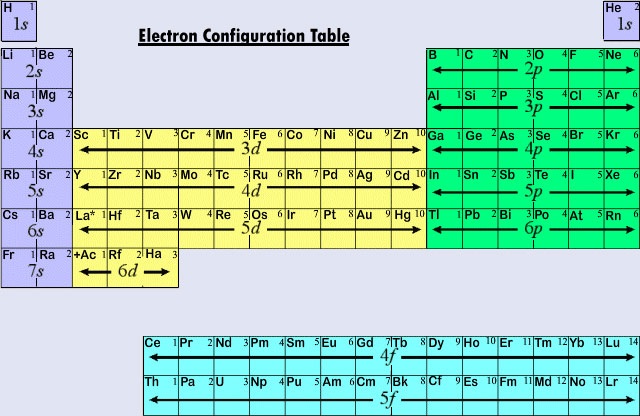
. The main body of the table is a 18 7 grid. Up to 2 2 s p 2 level 2. See Figure 1 Figure 1.
Quantum and the Periodic Table - Honors Chemistry Unit 2. View PatternsinValenceElectrons-1docx from CHM MISC at University of South Florida. Correlate the valence orbital of an atom with the atoms placement on the periodic table.
Write the electron configuration of an atom using the Aufbau Principle. They are in column 15 on the periodic table. Mg 1s 22s2 2p6 3s 2.
What is the electronic configuration of chlorine. So Oxygens electron configuration would be O 1s22s22p4. Chlorine is found in the group 17 the halogens on the periodic table.
What is the periodic table rows called. Filling of the s subshells. The History of the Modern Model of the.
So it makes sense that the structure of the periodic table reflects periodic trends in the electron configuration of elements. The periodic table is arranged in order of increasing atomic numbers. Therefore its ground state electronic.
To have a repeating pattern. Looking at the periodic table you can see that Oxygen has 8 electrons. Write the electron configuration for the first 20 elements of the periodic table.
For example all the electron configuration of all elements in group 2 can be expressed in the form X ns² where X is the configuration of the noble gas from the preceding period and n is the principal quantum number. The neutral atom chlorine Z17 for instance has 17 electrons. Scientists have identified three rules that explain the.
Electrons We will start to look at the periodic table by. There are two electrons in the s subshell and six in the p subshell. Periodic Table Electron Configuration Pattern.
The atomic number is the number of protons in the nucleus of an atom therefore it is the same to the charge number of the element. Periodic People Purpose To discover patterns from various kinds of information in order to arrange items or elements into meaningful sequences similar to the process by which early versions of the Periodic Table of Elements were constructed. Electronic configuration is also referred to as electron configuration.
Their electron configurations are 1 s 1 and 1 s 2 respectively. The atomic number of the elements on the periodic table are organized chronologically starting with Hydrogen with the the atomic number of 1 going from left to right. P 1s2 22s2 32p6 3s 3p 3.
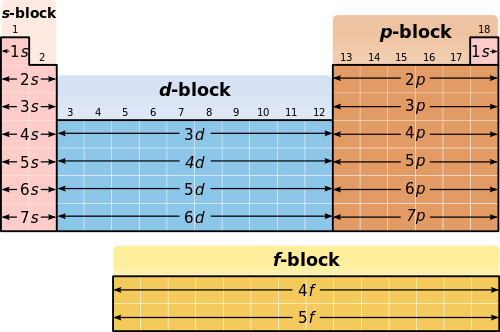
Elements are presented in increasing atomic number. The pattern of elements in the periodic table reflects the progressive filling of electronic orbitals. Up to 8 6 3 s p d 2 level 3.
There are exceptions to this. As you complete the activity keep the following in mind. The shape of the periodic table mimics the filling of the subshells with electrons.
Ge 1s 22s 2p63s 3p64s23d104p2 PART B SHORTHAND ELECTRON CONFIGURATION Use. Electron Configuration Li 1 3 1 1s2 2s1 N 5A 7 5 1s2 2s2 2p3 F 7A 9 7 1s2 2s2 2p5 Ne 8A 10 8 1s2 2s2 2p6 Na 1 11 1 1s2 2s2 2p6 3s1 Mg 2 12 2 1s2 2s2 2p6 3s2 Al 3A 13 3 1s2 2s2 2p6 3s2 3p1 Cl 7A 17 7 1s2 2s2 2p6 3s2 3p5 Ar 8A 18 8 1s2 2s2 2p6 3s2 3p6 K 1 19 1 1s2 2s2 2p6 3s2 3p6 4s1 Ca 2 20 2 1s2 2s2 2p6 3s2 3p6 4s2 Br 7A 35 7 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. One of the many patterns contained in the periodic table is that of electron configuration.
An electron configuration is the way that the electrons of an atom are arranged in the atomic orbitals. This gives them the Same electron configuration as a noble gas making them stable. We identified it from well-behaved source.
How many electron configurations does a chlorine atom have. Using the periodic table to determine the electron configurations of atoms is key but also keep in mind that there are certain rules to follow when assigning electrons to different orbitals electrons in a single orbital must have opposite spins electrons fill low principle energy levels first electrons are added by successively filling subshells spdf with electrons in a specific order. Chlorine has an electron configuration of 1s2 2s2 2p6 3s2 3p5.
Periodic Patterns in Electron Configurations In each column write the element name and the Electron. Click the links below for class notes andor worksheets. Later you will use these patterns to determine the order in which electrons fill the orbitals of an atom.
Its submitted by giving out in the best field. Your mission is to work with. Learn vocabulary terms and more with flashcards games and other study tools.
Introduction to the Periodic Table Activity 1. With He the n 1 shell is filled. Symbol e- Orbital Diagram and Longhand Electron Configuration 1.
The two columns on the leftthe alkali metals and alkaline earthsshow the addition of 1 and 2 electrons into s type subshells. Based on the order of fill above these 8 electrons would fill in the following order 1s 2s and then 2p. Quantum numbers Scientists experiments laws theories rules models of atom Electron configuration of atoms and ions Orbital notation Unit 2.
Period row Group column Use the table on your book cover which shows only. The answer is rather simple if you understand electron configurations. ANALYZING DATA Patterns in Electron Configurations As described by the quantum mechanical model of the atom every electron has a precise amount of energy and occupies a specific atomic orbital.
Periods 7 periods. The periodic table as an organisational tool to identify patterns and trends in and relationships between the structures including electronic configurations and atomic radii and properties including electronegativity first ionisation energy metallicnon-metallic character and reactivity of. The main properties that can be compared is the melting point ionization energy.
Unreactive due to electron configuration ns2np6 except He 1s2 Main group elements tend to gain or lose electrons to become isoelectronic same valence electron configuration as. The electron pattern for neon for instance is 1s 2 2s 2 2p 6. Metalloids have properties of metals and nonmetals.
Each new atom always repeats the pattern of the one before it. With an atomic number of ten neon has two electrons in the main shell and eight electrons in the second shell. In this activity you will identify these patterns.
Use the patterns within the periodic table to draw orbital diagrams and write longhand electron configurations for the following atoms. Predict the relative reactivity of an atom based on its electron configuration and placement on the periodic table. Principal Energy Level Sublevels Max.
If you consider the electronic configuration of an atom of each element in the Periodic Table you will see a number of patterns which are referred to as periodic trends or just trends. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers electron configurations and chemical properties. They are in column 14 of the periodic table.
Let us start with H and He. Start studying Periodic Table Chemical Bonds And Electron Configuration. Of electrons 1 s 2 level 1.
Up to 18. Elements with the same number of valence electrons are kept together in groups such. Atomic radii differ in an anticipated and logical way over the periodic table.
The names of groups and periods on the periodic chart are alkali metals alkaline earth metals transition metals halogens and noble gases. They have an ionic charge of 4- or 4 respectively. The Atom Patterns of the Periodic Table.
V 1s2 6 2s2 2p 3s2 3p6 4s23d3 4. You have been chosen for this top sec ret mission. Nitrogen can lose all 5 electrons resulting in a 5 charge.
Here are a number of highest rated Periodic Table Electron Configuration Pattern pictures on internet.
Electron Configurations And Magnetic Properties Of Ions Introduction To Chemistry

5 17 Electron Configurations And The Periodic Table Chemistry Libretexts

Electron Configurations And Magnetic Properties Of Ions Introduction To Chemistry

Electron Configurations In Atomic Energy Levels Video Lesson Transcript Study Com

Dublin Schools Lesson Electron Configurations Using The Periodic Table

Electron Configurations The Cavalcade O Chemistry

Electron Configuration And The Modern Periodic Table Examples Pedia

0 comments
Post a Comment